Recombinant single-domain antibodies
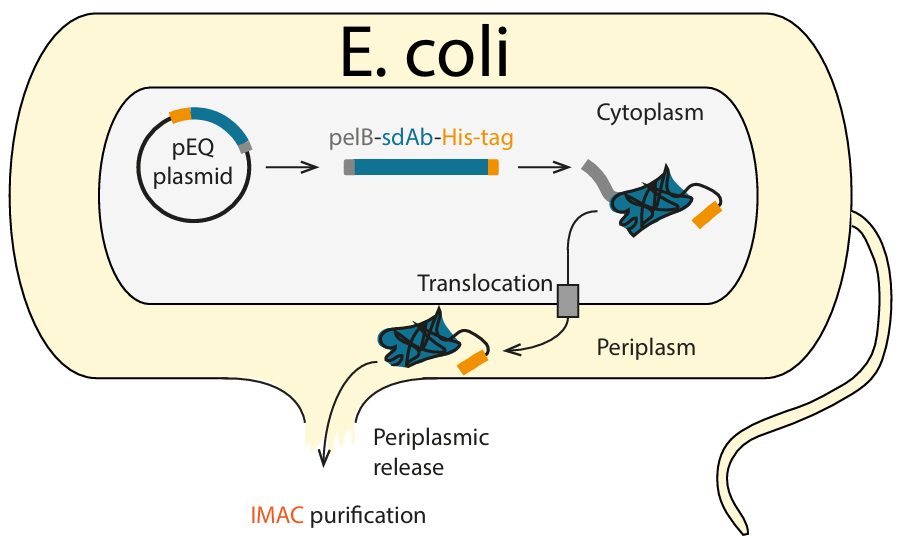
QVQ routinely produces sdAbs as recombinant proteins in E.coli. For this purpose, sdAbs are equipped with a leader sequence that directs them to the bacterial periplasm and is cleaved off in the process. From the periplasm, the sdAbs are then purified via a C-terminal His6-tag using immobilized metal affinity chromatography (IMAC).
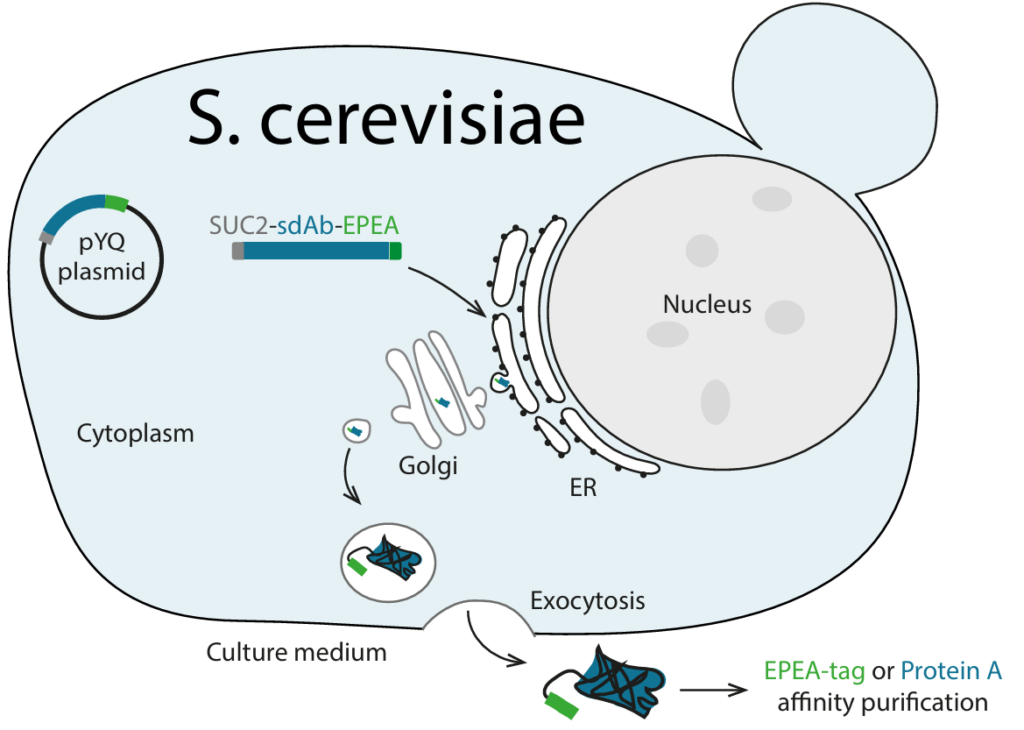
To increase production yields or to incorporate an extra cysteine for conjugation, sdAbs can also be produced in yeast (Saccharomyces cerevisiae). Here, the sdAbs are equipped with a signal peptide causing them to be secreted from the cells. As a consequence, the sdAbs can be purified from the culture medium using affinity chromatography. Approximately half of all sdAbs can be purified via protein A chromatography. The other half can be either purified via an EPEA sequence in our proprietary C-Direct tag or can be engineered to incorporate protein A binding affinity.
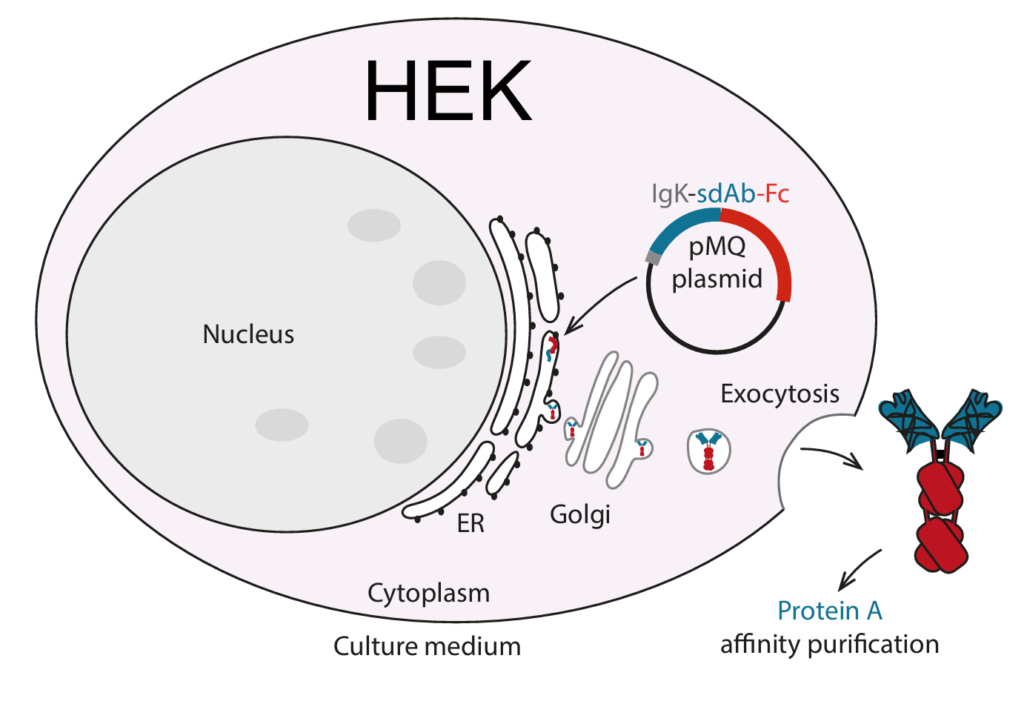
For some proteins, like sdAb-containing fusion constructs, expression in mammalian cells can be beneficial. For instance, sdAbs fused to Fc domains of IgG antibodies or multivalent sdAbs can be expressed in e.g. HEK293 or CHO-K1 cells with high yields. Furthermore, expression in mammalian cells can optimize the folding and post-translational modification of the expressed proteins.
After purification, all sdAbs are buffer-exchanged and quality controlled.
