Conjugation of single-domain antibodies
The production of sdAbs as recombinant proteins enables the straightforward incorporation of modifications for various functionalizations.
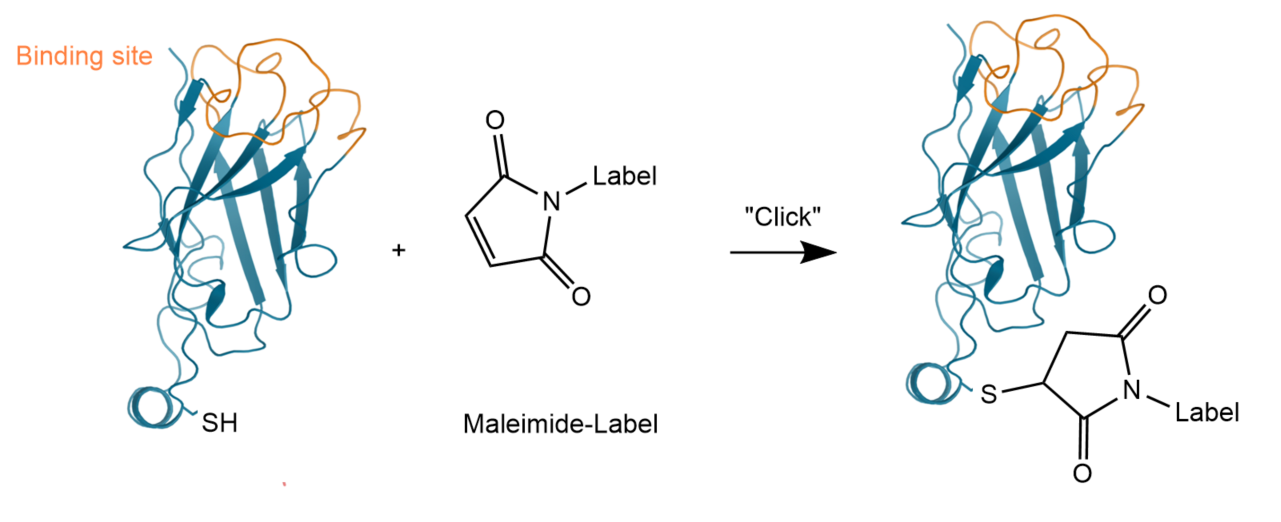
Different probes can be conjugated either randomly or in a site-directed manner. The random labeling approach is targeted towards lysines, which can negatively impact the sdAb’s affinity if lysines critical for antigen binding are labeled. Therefore, site-directed conjugation of sdAbs is often preferred with the conjugation site away from the binding site.
To this end, QVQ routinely equips sdAbs at their C-terminus with a proprietary C-Direct tag. This tag has low immunogenicity, allows for good expression levels in yeast, provides an affinity tag for purification, and contains an unpaired thiol for directional conjugation to a wide range of labels.
In solution, the thiol in the side chain of cysteines oxidizes to e.g. disulfide bonds. This leads to the formation of VHH dimers (~30% of the VHH population, can be determined by size-exclusion chromatography) or occupancy of the thiol by other groups like glutathiones. In both cases, the thiol side chain in these cysteines is unavailable for coupling to labels. Therefore, prior to conjugation, the cysteine-tagged sdAbs are treated with the reducing agent TCEP which reduces the cysteine in the C-direct tag, but not the internal disulfide bonds of the sdAb framework.
After this reduction, the now-reactive thiol group of the cysteine can be conjugated to maleimide-containing compounds in a thiol-Michael addition (thiol-maleimide reaction). This reaction is a type of “Click Chemistry” reaction as it is specific and high-yielding, works under simple conditions, and uses readily available reagents. The so-generated sdAb-conjugates carry one label per molecule and retain their binding affinity (label opposite to paratope).