Characterization of single-domain antibodies
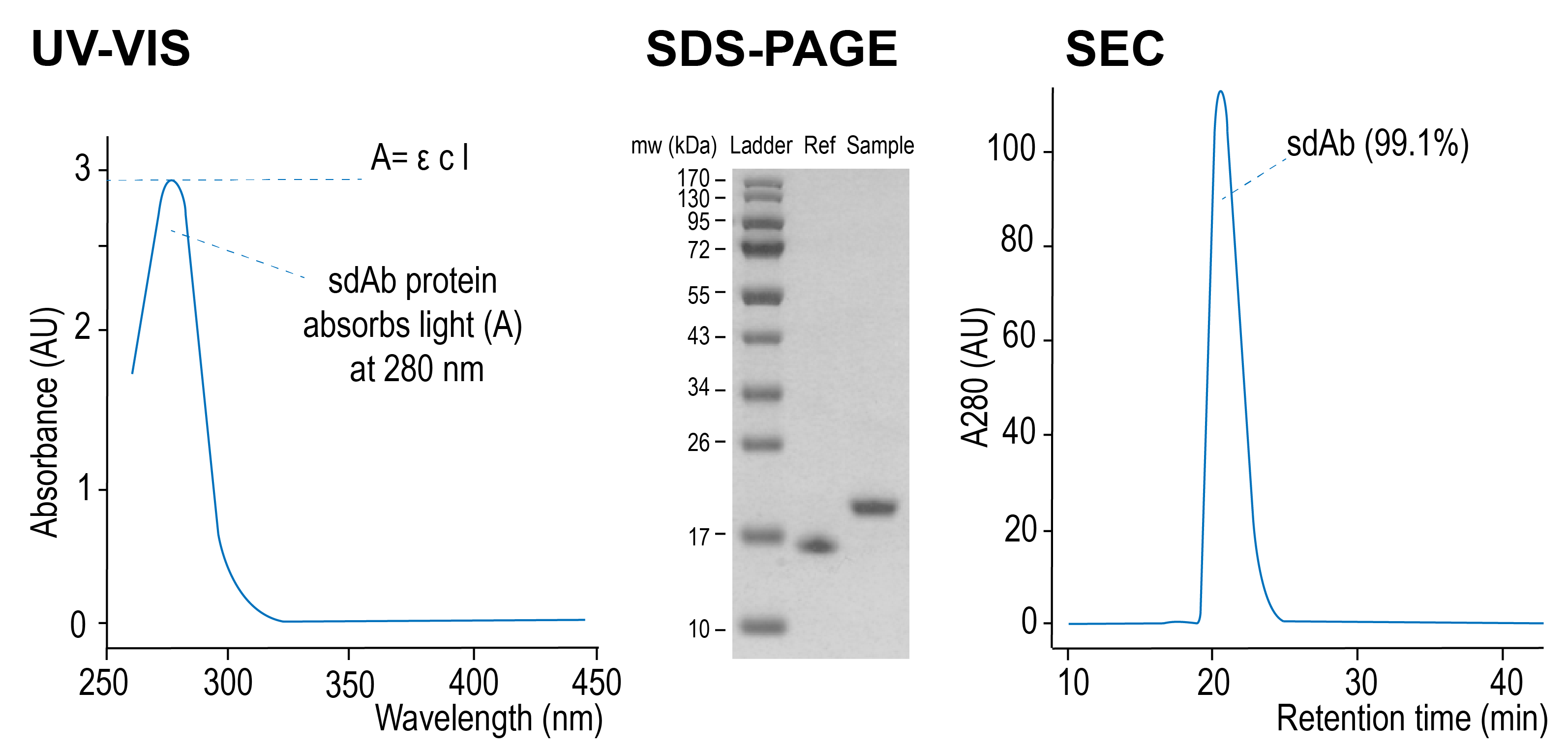
Standard quality control consists of UV-Vis spectroscopy and SDS-PAGE analysis for determination of protein concentration, integrity, and sample purity. Additionally, the composition of the native sample can be further analyzed by size-exclusion chromatography (SEC).
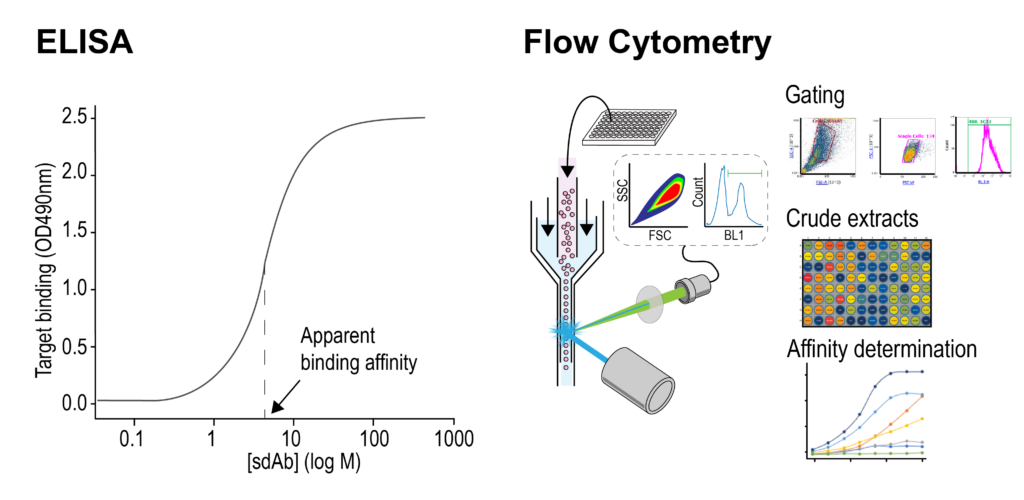
Antigen binding and apparent binding affinities of purified sdAbs are routinely determined by ELISA. QVQ also offers binding analyses on cells by cell-based ELISA or flow cytometry using an Attune Nxt.
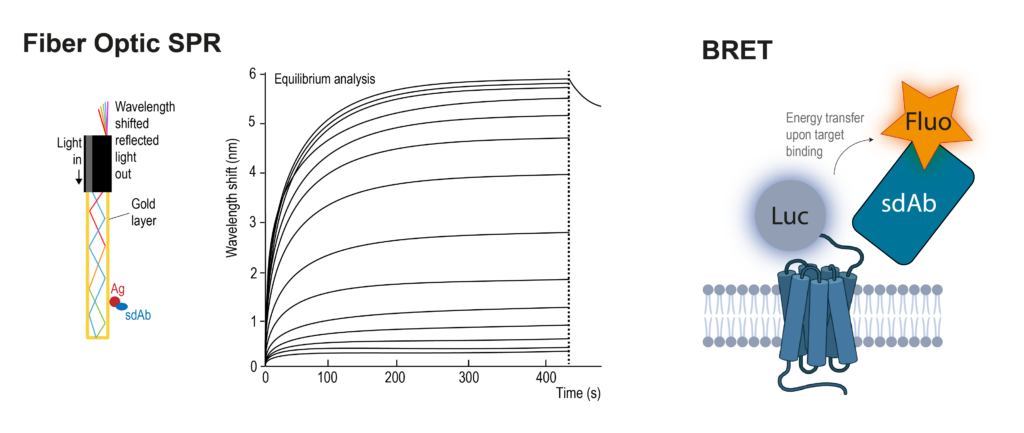
Equilibrium binding sensorgrams and binding kinetics can be assessed by surface plasmon resonance (SPR) using a fiber-optic-based SPR setup, or bioluminescence resonance energy transfer (BRET) using luciferase-tagged target proteins and fluorescently labeled sdAbs.
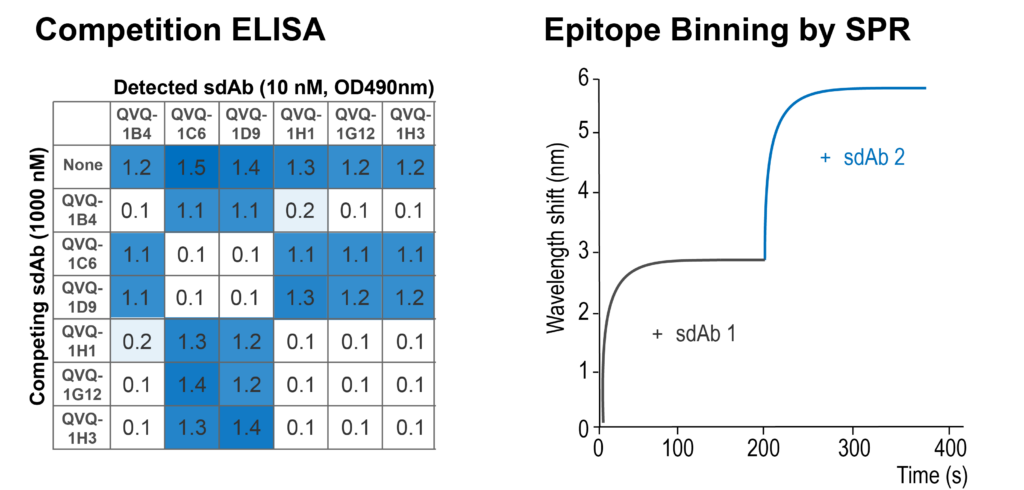
Competitive binding of lead molecules with other molecules, including sdAbs, antibodies, or other ligands can also be evaluated by ELISA or SPR, resulting in epitope bin information and IC50 values.
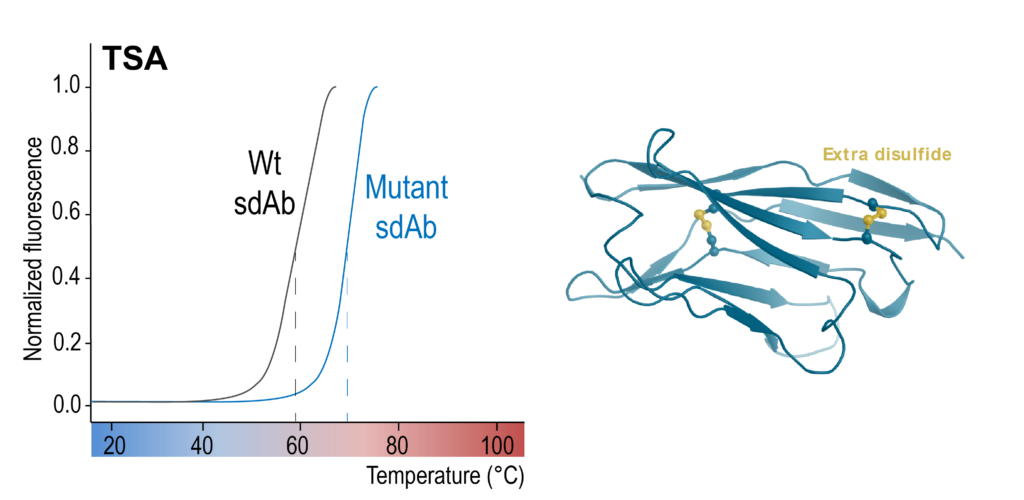
Both enzymatic and thermal stability of sdAbs can be assessed in-house. Enzymatic stability of sdAbs is assessed by SDS-PAGE and ELISA. Thermal stability can be assessed by Thermal Shift Assays (TSA) or Differential Scanning Fluorimetry (DSF).
Characterizations using other techniques can be performed in collaboration with partners. These include:
- Dynamic Light Scattering (DLS)
- Affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS)
- Hydrophobic interaction chromatography (HIC)
- Capillary Electrophoresis Sodium Dodecyl Sulfate (CE-SDS)
- Intact Protein Mass Spectometry (MS)
Please contact us for more information.
